Rheumatoid Arthritis
Applying our technology to combat drug non-response or resistance faced by rheumatoid arthritis patients
Rheumatoid Arthritis
The challenges being faced by RA patients
Rheumatoid arthritis (RA) is a chronic inflammatory disease in which hyperactivated immune cells induce maladaptive persistent inflammation in the joints, leading to synovial inflammation and bone remodeling. In the US, RA currently affects roughly 1% of the population and carries a total annual societal cost burden of approximately $39.2 billion. In RA, sustained elevations of pro-inflammatory cytokines elicit chronic tissue damage and pain, which ultimately leads to loss of mobility and significant impairment of the patient’s lifestyle.
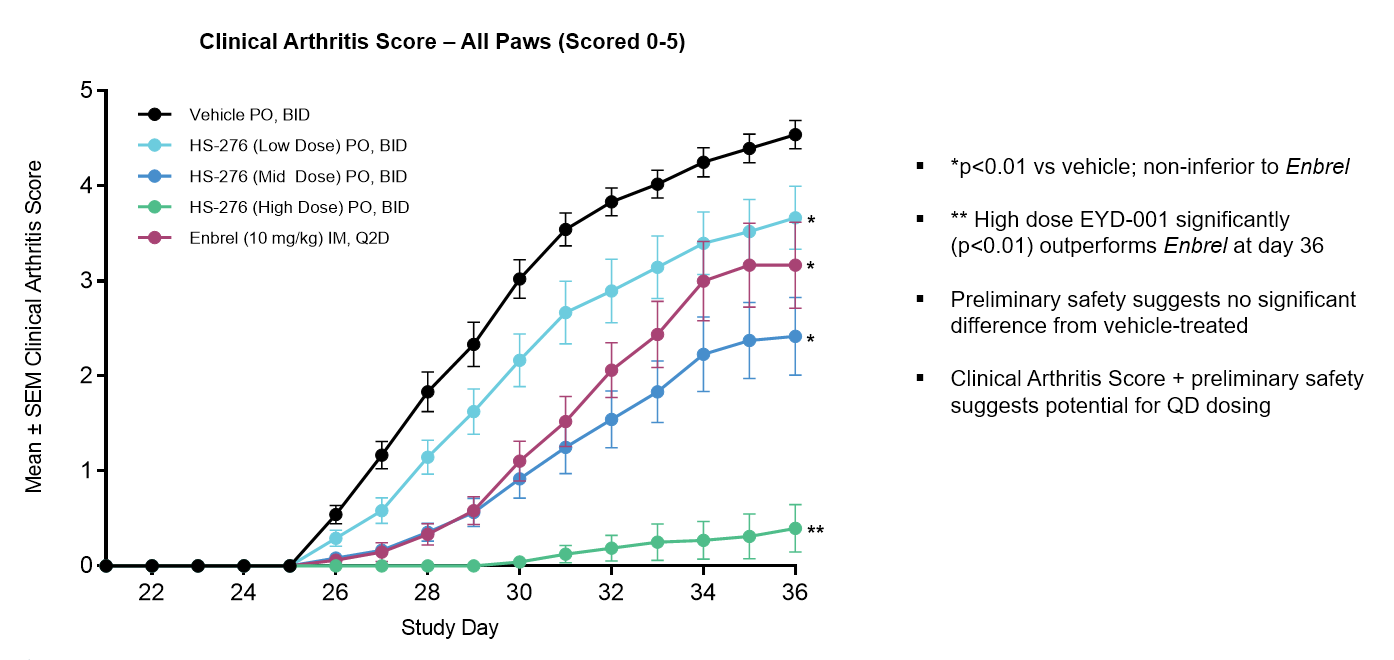
Development and approval of anti-tumor necrosis factor (anti-TNF) biological therapies (e.g., Humira, Enbrel, and Remicade) has transformed treatment options for patients with moderate to severe RA. However, over 40% of patients taking anti-TNF biologics either fail to respond to initial treatment or lose response over time, leading to the need for experimental cycling through other anti-TNFs or JAK inhibitors to manage disease. However, due to cytokine oversuppression, all of these therapies suffer from serious safety issues including risks of infections, malignancies, cardiotoxicities and blood clots. Thus, there is a clear need for a novel, potent and highly selective, orally bioavailable TAK1 inhibitor that affords RA patients the benefits of anti-TNF therapy without the shortcomings of biologics, coupled with a superior safety profile through restoration of self-tolerance versus immune oversuppression.
Over 40% of patients fail to
respond, or lose response over time, to conventional
antibody-based therapies
This result is due mainly to the formation of anti-drug
antibodies
Solution: Development of
orally bioavailable small
molecule inhibitors
of TAK1
How EYD-001 can be applied
Working to combat drug resistance and non-response among RA patients
Unlike biologic-based therapies, small molecule drugs are not immunogenic and thus do not elicit an immunological response to the drugs themselves. This avoidance allows for a more targeted treatment regimen with a larger therapeutic window. Additionally, dosing paradigms can be more easily adjusted within the therapeutic range for maximum efficacy. As a result, as opposed to anti-TNF injections or infusions, EYD-001's target product profile aims to provide patients with highly effective once-a-day oral dosing coupled with a superior safety profile.
Benefits of Our
Application
Orally bioavailable small molecule therapeutic, novel MOA, highly potent and exquisitely selective anti-TNF
Selective targeting of
TNF signaling provides for a larger therapeutic window, superior efficacy, improved safety (i.e., immune/TNF dampening vs oversuppression)
Customized dosing
regimens which are more easily adjustable to fit patients' needs
Additional information and resources
Our Technology
First selective orally bioavailable inhibitors of TAK1 for the treatment of RA
Our Company
EydisBio is a small biotech located in Durham, North Carolina. Originally created as a spinout of Duke University, EydisBio has gone on to become a leader for the development of selective and potent small molecule inhibitors of disease relevant kinases.
Our Team
Our team is composed of experienced pharmacologists, chemists, and biologists who leverage their years of industry experience to advance small molecule inhibitors into clinics.
"*" indicates required fields